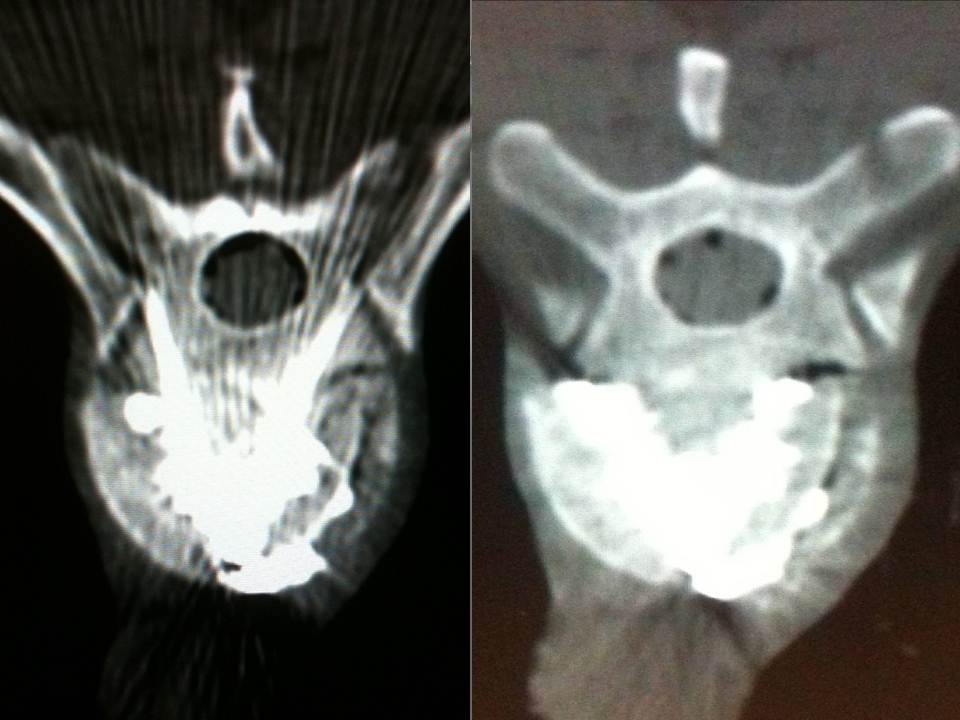
- Axial CT image. A skin entry point was determined, and the distance was measured from the midline
- The trocars are advanced successively under monitoring by CT slices and lateral fluoroscopy. We use 13-gauge trocars and insert them manually.(13-gauge 10-cm Trocar t’am (Thiebaud, Thonon-les-Bains, France)
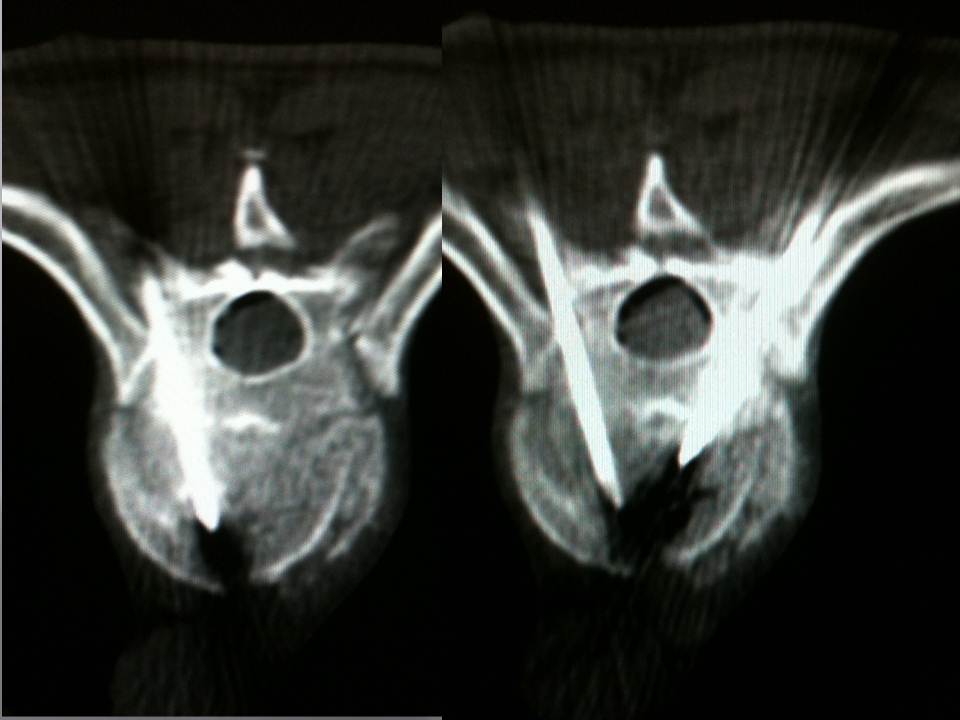
- Axial CT images confirming the good progression of the trocars. We transfix the fracture line with the two trocars in bilateral and symmetric manner, with their ends situated medially in order to limit lateral leaks.
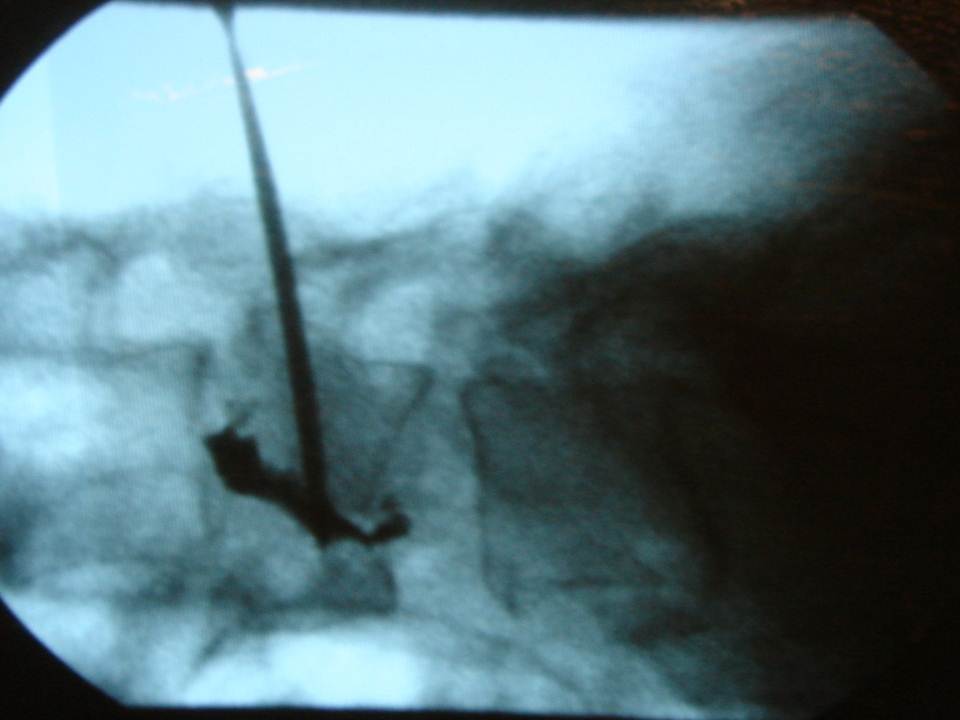
- the cement is injected at the level of the anterior fragment of the fracture, then the trocars are withdrawn very slowly to the fracture level while cement is injected progressively
- The diffusion of the cement into the fracture line in real time is monitored by lateral fluoroscopy
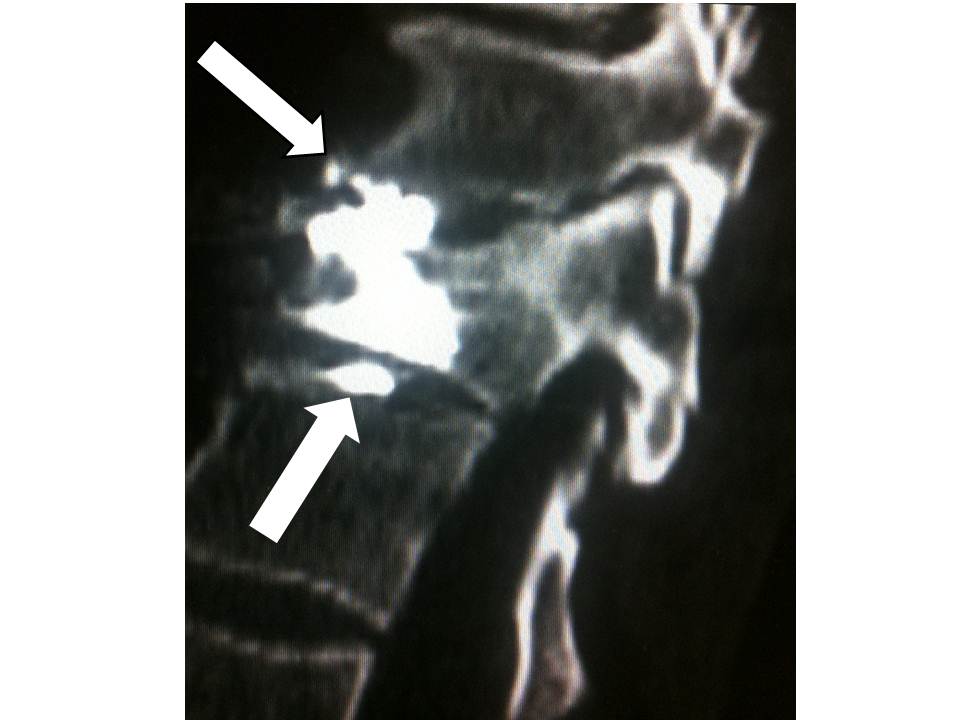
- Discal leaks were noted (14%) , with no significant consequences for the clinical course, particularly for the vertebrae immediately above and below
Vertebral split A2 fracture: the role of vertebroplasty.
Abstract
Objective
The aim of this study was to evaluate CT- and fluoroscopy-guided percutaneous vertebroplasty in the management of split burst fracture (Magerl A2).
Materials and Methods
Institutional review board approval and informed consent were obtained for this study. Fifty consecutive adult patients were prospectively treated by an interventional radiologist who made a percutaneous vertebroplasty under CT and fluoroscopy guidance. All these procedures were performed under local anesthesia. The post-operative follow-up ranged from 12 to 24 months.
Results
The results obtained match those of the literature in the area of hyperalgesic osteopoenic fractures: The visual analogue scale and Oswestry disability index decreased from 7.5 +/- 1.5 to 2.1 +/- 1.2 and from 65.3 +/- 16.2 to 16.1 +/- 5.0 respectively. All of the results were stable in time and in particular over the long term, at 1 year and 2 years.
Conclusion
Vertebroplasty offers an intermediate alternative to the traditional treatments.The results of our study showed that non-neurologic A2 split fracture could be successfully treated by a percutaneous vertebroplasty under CT and fluoroscopic guidance with an excellent long-term outcome. The brief hospitalization and the speedy return to active life have very interesting economic and social consequences.
Keywords: Vertebroplasty. Burst fracture.Split fracture. Magerl A2.Local anesthaesia. CT and fluoroscopy guidance. Interventional radiology
The percutaneous vertebroplasty procedure was performed for the first time in 1984 by the team of Professor Deramond at Amiens University Hospital Centre in France (1-2). It consists of injecting acrylic cement into the vertebral body in order to consolidate it. The original indication was that of aggressive angioma. The indications have been gradually broadened to include vertebral metastatic disease and then hyperalgesic osteoporotic fractures(3-11). The evolution of the equipment and of the experience of interventional radiology teams has made it possible to expand the group of indications to include stable traumatic fractures. The objective of the present article is to describe the vertebral consolidation techniques used to treat patients whose fracture lesions are within the limits of the indications, especially the A2 fractures of the Magerl classification (12). Different techniques are necessary in order to improve the safety of the procedure, while a learning curve and good mastery of simple vertebroplasty are imperative before any attempt to use these more delicate vertebroplasties.
Equipment and method:
All procedures in this study were approved by the Institutional Review Board of our institution. Patients were enrolled after giving written informed consent.
From january 2004 to october 2010 we realised a Single-centre prospective study of 50 consecutive patients without neurologic deficit referred by the trauma departments of our institution for post-traumatic vertebral fracture of class A2 according to the Magerl classification. Procedures were decided only following an interdisciplinary meeting between interventional radiologists and orthopedic surgeons. Step-by-step volume CT over the entire spine made it possible to establish a lesion assessment and to classify the vertebral fracture as split fracture of grade A2 in the Magerl classification.
Description of the technique:
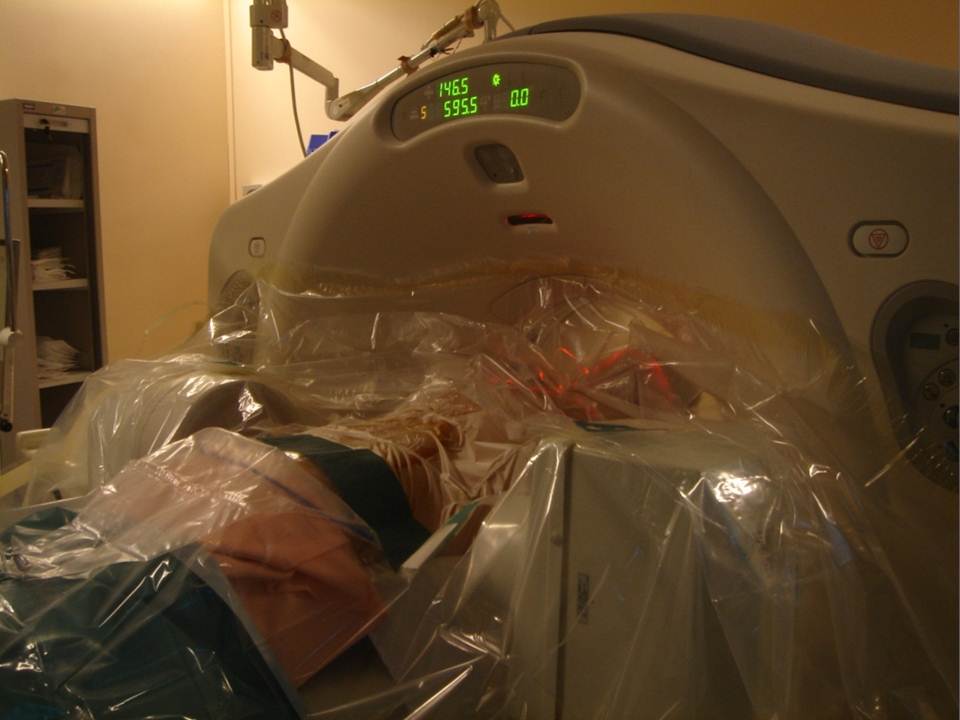
All patients were treated by a senior interventional radiologist (with ten years of experience). After haemostatic control, procedures were performed under surgical conditions of aseptia in an interventional CT room using CT (GE Lightview 8-row MDCT scanner; GE Healthcare, Waukesha, Wis, USA) and lateral fluoroscopy (GE Stenoscop C-arm) guidance. Patients were placed in a prone position on the CT table with a support optionnaly placed under abdomen to decrease lumbar lordosis (figure 1).The common denominator in all of the typical cases described is a dual guidance system using CT and fluoroscopy to facilitate positioning of the trocar by better visualisation of vertebral structures and lesions to be treated as well as to monitor vertebral filling in real time to limit cement leaks (13-14). These procedures are performed in a dedicated interventional CT suite under conditions of surgical asepsis. In order to analyse the vertebral level to be consolidated, a preoperative MRI examination is necessary in order to visualise a T2-weighted vertebral hypersignal sequence with suppression of the fat signal (fat sat or stir). This reveals oedema secondary to the corporeal lesion.
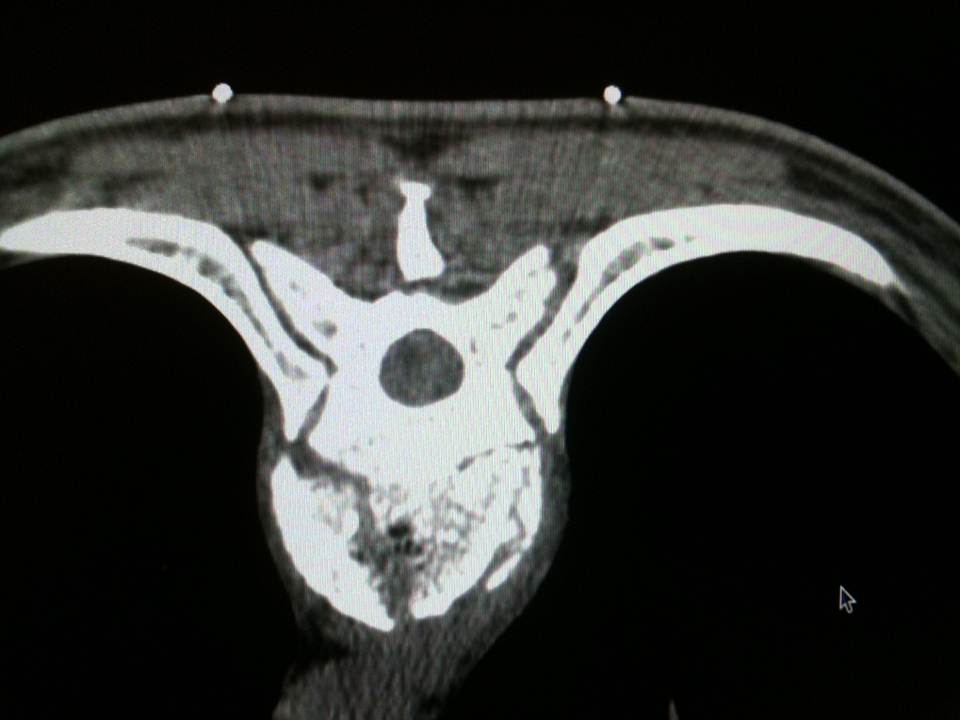
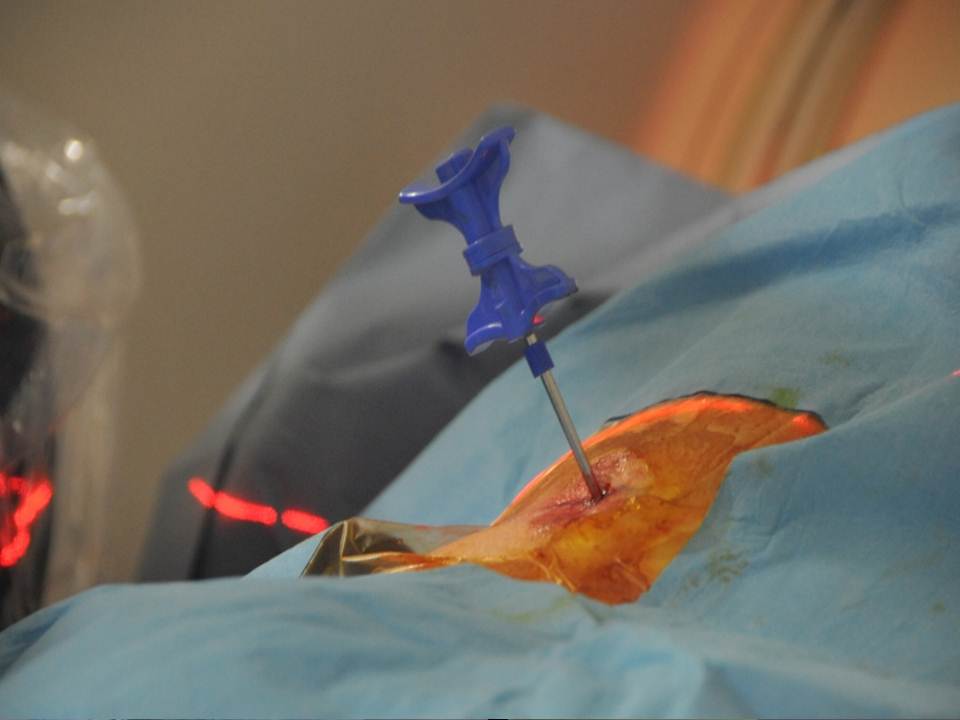
The multiplanar reconstructions make it possible to locate the level to be treated as well as the entry route. The entry route is calculated on the axial slices, thus permitting a transpedicular, intercosto-vertebral or extra-vertebral approach depending on the level and type of lesion to be treated radio-opaque dot is positioned on the skin as a function of the location established on the axial CT slices and is then confirmed by a new scan (figure 2). Preparation of the equipment necessitates: a sterile table drape, a sterile perforated drape (used for angioplasties), a 22-gauge needle for subcutaneous anaesthesia, a bottle of 1% xylocaine (lidocaïne 1% Xylocaïne; Astra, Sodertalge, Sweden), a scalpel and two 13-gauge trocars for guided vertebroplasty and vertebral biopsy (t’am,Thiebaud, Thonon-les-Bains, France). All procedures are performed under local anaesthesia according to the same protocol: Local anaesthesia is induced by subcutaneous injection of 3 cc of 1% xylocaine, then 3 minutes later the 20-cm 20-gauge Chiba needle is inserted to the point of contact with the periostium under fluoroscopic guidance. Monitoring by CT in step-by-step mode is used to confirm proper positioning of the Chiba needle. A further 3 cc (approximately) of 1% xylocaine is injected at the vertebral entry point to induce anaesthesia of the periostium. The break-off end of the Chiba needle is withdrawn, leaving space for a true guide, into which the perforated trocar is inserted as far as bone contact and in particular the precise level of the anaesthesia (figure 3). The advance of the trocar into the vertebral body is performed under iterative fluoroscopic and CT guidance (figure 4). Proper final positioning of the distal end of the trocar in the zone to be treated can be confirmed by CT monitoring. The cement is prepared by means of the closed-loop mixer, in order to avoid vapour releases. The 1-cc Luer Lock syringes are filled with cement in its early liquid phase. We use a radio-opaque cement (Osteo-firm Radiopaque bone cement; Cook, Bloomington, IN, USA) in which we mix 4 grams of tungsten to make it highly radio-opaque. This high radio-opacity makes visualisation perfect during vertebral filling, thus constituting an additional safety element (figure 5-6). The cement used must have time-variant viscosity phases, from its fluid until its pasty phase.
Statistical analysis:
Analysis of variance of the results was carried out by using the Levene test; statistical significance was analyzed with the Pillai-Spur, Wilks-Lambda, Hotelling-Spur and Scheffe multivariable tests.
The statistical significance hypothesis was accepted for p< 0.05.
The numbers and percentages are qualitative values. The average, median, and standard deviation are quantitative values. An analysis of absolute values was performed; to judge the variation in VAS over time. We calculated the differences in VAS for each patient at different times T as compared to the initial VAS value and performed an analysis on the relative differences. The Levene tests demonstrate that the data can be analyzed for statistical significance with a significance threshold starting at 5%. We tested these differences as compared to the null hypothesis by means of a Wilcoxon signed ranks test. SPSS 7-0 was used to perform statistical analysis.
Results:
32 men and 18 women aged from 28 to 72 years (mean: 48 years) received this procedure. 35 had suffered traffic accidents, 5 falls from horseback, 6 skiing accidents, 1 a water-skiing accident and 9 falls from windows or balconies. From start to finish, the duration of the vertebroplasty varied from 30 to 50 minutes per patient, with a mean of 42 minutes. The filling of the vertebral body was satisfactory in 100% of the patients, extending to the level of the fracture line. No neurological or infectious complication was observed. We did note 7 discal leaks (14%) (fig 7), but these had no significant consequences for the clinical course, particularly for the vertebrae immediately above and below. Clinical and radiological follow-up examinations were performed on day 2 and at 1 month, 6 months and one year. The results obtained match those of the literature in the area of hyperalgesic osteopoenic fractures: The visual analogue scale and Oswestry disability index decreased from 7.5 +/- 1.5 to 2.1 +/- 1.2 and from 65.3 +/- 16.2 to 16.1 +/- 5.0 respectively. All of the results were stable in time and in particular over the long term, at 1 year and 2 years. In all cases, CT monitoring revealed satisfactory consolidation of the fracture line.
Discussion
Vertebroplasty for split burst fracture is a delicate procedure in many respects. The fracture separates the vertebral wall into 2 parts and may cause collapse of the posterior wall. This procedure is still within the limits of the indications of vertebroplasty, but the decision to proceed must be made in agreement with the neurosurgical or orthopaedic team. Numerous papers attest to the efficiency of this procedure and of the low rate of complications (15-21). In our experience, the coupling of CT and fluoroscopy is fundamental. The axial slices permit perfect visualization of the ends of the trocars precisely within the vertebral fracture. The fluoroscopic guidance verifies that the trocars are at mid-height of the vertebra. The trocars are advanced successively under monitoring by CT slices and lateral fluoroscopy. We use 13-gauge trocars and insert them manually. We also avoid the use of a surgical hammer, so as to limit the risk of displacement of bone fragments. We transfix the fracture line with the two trocars in bilateral and symmetric manner, with their ends situated medially in order to limit lateral leaks. While in its pasty phase, the cement is injected at the level of the anterior fragment of the fracture, then the trocars are withdrawn very slowly to the fracture level while cement is injected progressively, in order to create a cement bridge between the anterior fragment and the fracture line. A CT control verifies that the end of the trocar is situated perfectly in the fracture. The cement is injected slowly into the cannula then pushed with the needle of the trocar. The diffusion of the cement into the fracture line in real time is monitored by lateral fluoroscopy. The axial CT slices confirm the good diffusion into the fracture and the absence of para-vertebral leaks. The trocars are withdrawn progressively, while cement is injected into the cannula by means of the needle. The patients are allowed out of bed on the next day, and they do not have to wear a corset. Mobilization began on the 1st or 2nd postoperative day. A soft brace is worn out of bed for 3 months to restrict gross motions. Patients are instructed how to move “en bloc” and encouraged to walk; no physical therapy is performed during the first 2 months. Rigorous clinical and radiologic follow-up is necessary in order to detect any neurological signs of compression and to appraise the vertebral consolidation. The traditional treatment of this type of fracture is either conservative, involving the wearing of a corset, or surgical, with posterior arthrodesis(22-25). These two techniques have their own disadvantages in terms of consolidation time and risk of pseudarthrosis as well as postoperative risks of infection or functional impairment due to attack of the para-vertebral muscle masses and of the presence of the arthrodesis material. Vertebroplasty offers an intermediate alternative to the traditional treatments. Percutaneous vertebroplasty is a vital procedure in the therapeutic arsenal for vertebral fractures. The evolution of the equipment and of the guidance mode makes it possible to perform this procedure under optimum conditions of safety and ease. Numerous expert centers have chosen this technical development because of the clear improvement of the tolerance for this procedure as well as of the speed, ease and safety of the operation. The brief hospitalization and the speedy return to active life have very interesting economic and social consequences.[toggle title="REFERENCES"]1.Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty.Neurochirurgie.1987;33(2):166-8
2. Franc J, Lehmann P, Saliou G, Monet P, Kocheida EM, Daguet E, Laurent A, Legars D, Deramond H. Vertebroplasty: 10 years clinical and radiological follow-up.J Neuroradiol. 2010 Oct;37(4):211-9
3. Fransen H. Minimal invasive treatment of vertebral compression fractures: vertebroplasty.Neuroradiology. 2011 Sep;53 Suppl 1:S199-201.
4. Muijs SP, van Erkel AR, Dijkstra PD. Treatment of painful osteoporotic vertebral compression fractures: a brief review of the evidence for percutaneous vertebroplasty.J Bone Joint Surg Br. 2011 Sep;93(9):1149-53
5. Klazen CA, Lohle PN, de Vries J, Jansen FH, Tielbeek AV, Blonk MC, Venmans A, van Rooij WJ, Schoemaker MC, Juttmann JR, Lo TH, Verhaar HJ, van der Graaf Y, van Everdingen KJ, Muller AF, Elgersma OE, Halkema DR, Fransen H, Janssens X, Buskens E, Mali WP. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial.Lancet. 2010 Sep 25;376(9746):1085-92
6. Brodano GB, Amendola L, Martikos K, Bettuzzi C, Boriani L, Gasbarrini A, Bandiera S, Terzi S, Greggi T, Boriani S. Vertebroplasty: benefits are more than risks in selected and evidence-based informed patients. A retrospective study of 59 cases.Eur Spine J. 2011 Aug;20(8):1265-71
7. Anselmetti GC, Manca A, Montemurro F, Hirsch J, Chiara G, Grignani G, Carnevale Schianca F, Capaldi A, Rota Scalabrini D, Sardo E, Debernardi F, Iussich G, Regge D.Percutaneous vertebroplasty in multiple myeloma: prospective long-term follow-up in 106 consecutive patients.Cardiovasc Intervent Radiol. 2012 Feb;35(1):139-45 2012
8. Saliou G, Kocheida el M, Lehmann P, Depriester C, Paradot G, Le Gars D, Balut A, Deramond H. Percutaneous vertebroplasty for pain management in malignant fractures of the spine with epidural involvement.Radiology. 2010 Mar;254(3):882-90.
9. Lee B, Franklin I, Lewis JS, Coombes RC, Leonard R, Gishen P, Stebbing J.The efficacy of percutaneous vertebroplasty for vertebral metastases associated with solid malignancies.Eur J Cancer. 2009 Jun;45(9):1597-602
10. Mont'Alverne F, Vallée JN, Guillevin R, Cormier E, Jean B, Rose M, Caldas JG, Chiras J.Percutaneous vertebroplasty for multiple myeloma of the cervical spine.Neuroradiology. 2009 Apr;51(4):237-42
11. McDonald RJ, Trout AT, Gray LA, Dispenzieri A, Thielen KR, Kallmes DF. Vertebroplasty in multiple myeloma: outcomes in a large patient series.AJNR Am J Neuroradiol. 2008 Apr;29(4):642-8. Epub 2008
12. Margel F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprensive classification of thoracic and lumbar injurues. Eur Spine j.1994;3:184-201.
13. Gangi A(1994) Percutaneous vertebroplasty guided by a combination of CT and fluoroscopy.AJNR. Am J Neuroradiol. 15(1):83-6.
14. Amoretti N, Marcy PY, Lesbats-Jacquot V, Hovorka I, Fonquerne ME, Roux C, Hericord O, Maratos Y, Euller-Ziegler L.Combined CT and fluoroscopic guidance of balloon kyphoplasty versus fluoroscopy-only procedures.Skeletal Radiol. 2009 Jul;38(7):703-7
15.Huet H et al.Burst-fractures and cementoplasty. J Neuroradiol.2005 Jan;32(1):33-41.
16. Li CH, Chang MC, Liu CL, Chen TS. Osteoporotic burst fracture with spinal canal compromise treated with percutaneous vertebroplasty.Clin Neurol Neurosurg. 2010 Oct;112(8):678-81
17. Amoretti N, Hovorka E, Marcy PY, Lamasse C, Brunner P, Roux C, Chevallier P, Boileau P, Bruneton JN. Burst fracture of the spine involving vertebrae presenting no other lesions: the role of vertebroplasty.Clin Imaging. 2005 Nov-Dec;29(6):379-82
18. Verlaan JJ, Dhert WJ, Oner FC. Vertebroplasty for burst fractures.J Neurosurg Spine. 2005 Mar;2(3):398-9
19. Chen JF, Lee ST. Percutaneous vertebroplasty for treatment of thoracolumbar spine bursting fracture.Surg Neurol. 2004 Dec;62(6):494-500
20. Chen JF, Wu CT, Lee ST.Percutaneous vertebroplasty for the treatment of burst fractures. Case report.J Neurosurg Spine. 2004 Sep;1(2):228-31
21.Nakano M, Hirano N, Matsuura K, Watanabe H, Kitagawa H, Ishihara H, Kawaguchi Y.Percutaneous transpedicular vertebroplasty with calcium phosphate cement in the treatment of osteoporotic vertebral compression and burst fractures.J Neurosurg. 2002 Oct;97(3 Suppl):287-93
22. Schmid R, Lindtner RA, Lill M, Blauth M, Krappinger D. Combined posteroanterior fusion versus transforaminal lumbar interbody fusion (TLIF) in thoracolumbar burst fractures.Injury. 2012 Jan 6
23. Schmid R, Krappinger D, Seykora P, Blauth M, Kathrein A. PLIF in thoracolumbar trauma: technique and radiological results.Eur Spine J. 2010 Jul;19(7):1079-86
24. Rajasekaran S.Thoracolumbar burst fractures without neurological deficit: the role for conservative treatment.Eur Spine J. 2010 Mar;19 Suppl 1:S40-7
25. Blondel B, Fuentes S, Metellus P, Adetchessi T, Pech-Gourg G, Dufour H.Severe thoracolumbar osteoporotic burst fractures: treatment combining open kyphoplasty and short-segment fixation.Orthop Traumatol Surg Res. 2009 Sep;95(5):359-64[/toggle]