Inhalation study of methyl methacrylate following radiologist exposure during percutaneous vertebroplasty
Abstract
Objective
To assess the atmospheric concentrations of methylmethacrylate (MMA)vapors during percutaneous vertebroplasty for the interventional radiologist and the other operating room staff.
Materials and methods
During percutaneous vertebroplasty, a polymethylmethacrylate(PMMA) mixture (about 20 mL) was prepared with a mixing system in a normally ventilated room. Atmospheric concentrations of MMA vapors were measured by a GABIE(Gas Absorbent Badges for Individual Exposure) passive sampler attached to the surgical gowns of the interventional radiologist and the other operating room staff over aperiod of 460 min. Active sampling was performed during 15 min withan individual pump placed near the breathing zone of the radiologist. MMA vapour concentrations were then measure dusing gas chromatography and activated charcoal tubes.
Results
Mean MMA vapour concentrations measured by theGABIE badges over the period of 460 min were 0.51 parts per million (ppm) for the radiologist and 0.22 ppm for the other operating room staff. The emission peaks measured by using charcoal tubes during 15 min were 3.7 ppm.
Conclusion
MMA vapor concentrations during percutaneous vertebroplasty were well below there commended maximum exposure of 100 ppm over the course of an 8-hour workday.
Key words:methylmethacrylate; active sampling; passive sampling; exposure; vertebroplasty.
Introduction
Percutaneous vertebroplasty is a procedure that involves an injection of polymethylmethacrylate (PMMA)into a diseased vertebral body under fluoroscopy and/or CT guidance. Since the first procedure in 1984[1], vertebroplasty has gained wide spread popularity for the treatment of painful vertebral compression fractures which maybe secondary to osteoporosis, osteolytic metastases and multiple myeloma[2, 3].
However, during these interventions, the operating room staff is exposed to potentially toxic methylmethacrylate(MMA)fumes[4-6]. MMA, the methyl ester of 2-methylpropenoic acid [CH2 =C(CH3)COOCH3], is a colorless liquid and a widely used monomer in medicine[7-10]. MMA is used frequently during neurosurgical, orthopaedic surgical, and interventional radiologic procedures as “bonecement” to fillspaces in bones.
The risk of toxic vapors to the operating room personnel has been discussed by Wesley and Brinsko[11]; the liquid monomer used in the mixture can escape into the air, causing a sharply noxious odor.
Experimental and clinical studies have documented that monomers may cause a wide range of adverse health effects such as irritation to skin, eyes, and mucous membranes, allergic dermatitis, stomatitis, asthma,neuropathy, disturbances of the central nervous system, liver toxicity, and changes in blood parameters[7, 8, 11-15].
The most important exposure route of MMA is by inhalation[7]. It has been shown that the lungs are the primary organs for the clearance of MMA. Many experimental studies have shown alterations of the respiratory system of animals exposed to MMA. Thushypocapnia, hypercapnia, acidosis, pulmonary
oedema, haemorrhage, congestion, and necrosis, and Cheyne-Stokes dyspnea have beendescribed[5, 6, 16, 17]. In humans, functional disorders such as cough, sore throat, respiratory irritation, and occupational asthma have been noted[18-20].
To the best of ourknowledge, very few studies have been published on the level of exposure of the operating room staff to MMA fumes during vertebroplasty[21]. The purpose of this study was to assess the atmospheric concentrations of MMA vapors during percutaneous vertebroplasty for the interventional radiologist and the other operating room staff.
Materials and methods
ThePMMA mixture (about 20 mL) used for percutaneous vertebroplasty was prepared with a mixing system (M’NX, Thiebaud, Thonon-les-Bains, France) on a workbench without the use of a fume hood or other vapour control device.
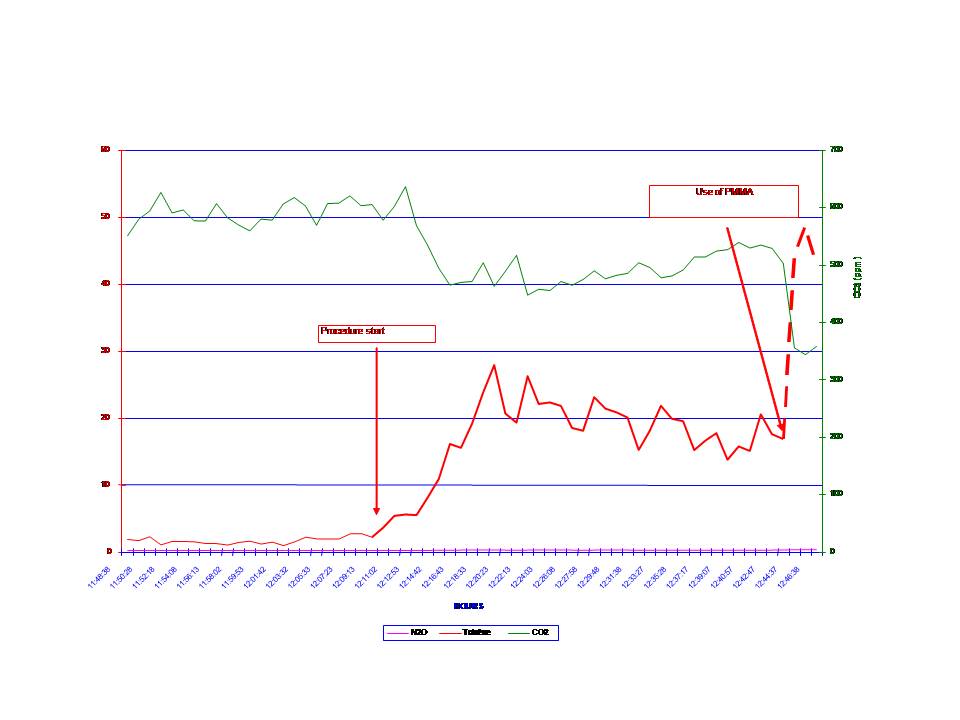
Pollutant concentrations in the operating theatre were accurately known using an infrared spectroscopic detector which measured two weeks before organic compounds vapors and CO2concentrations.
Atmospheric concentrations of MMA vapors were measured continuously with a GABIE(Gas Absorbent Badges for Individual Exposure)passive sampler attached to the surgical gowns of the interventional radiologist (involved in mixing and injecting PMMA)and the other operating room staff (two radiologic technologists) over a period of about 460 min. The GABIE badge (Fig.2) was developed by the INRS(Institute for Radiological Protection and NuclearSafety, Paris, France). This passive individual collection badge makes it possible to simultaneously sample a multitude of organic gases and vapors in order to determine the average exposure values.
Two GABIE badges were also positioned on the CT scan and near the filtering system (Fig. 3)of the operating room to measure MMA vapors concentrations in these locations.
This passive sampling allows to measure the long-term exposure limit values (defined by INRS) which are measured over a working period of 8 hours. These values are intended to protect personal from deferred effects of MMA.
Moreover, active sampling was performed using an individual air-sampling pump which was placed near the breathing zone of the radiologist (Fig.4). The samples were collected during a period of 15 min (corresponding to the mixing and injection of PMMA). MMA vapour levels in the samples were then quantified using gas chromatography(coupled with infrared spectroscopic detector) and activated charcoal tubes. This active sampling allows to measure the short-term exposure limit values (defined by INRS) which are measured over a reference period of 15 minutes. These values are intended to avoid toxic effects due to exposure peaks.
Results
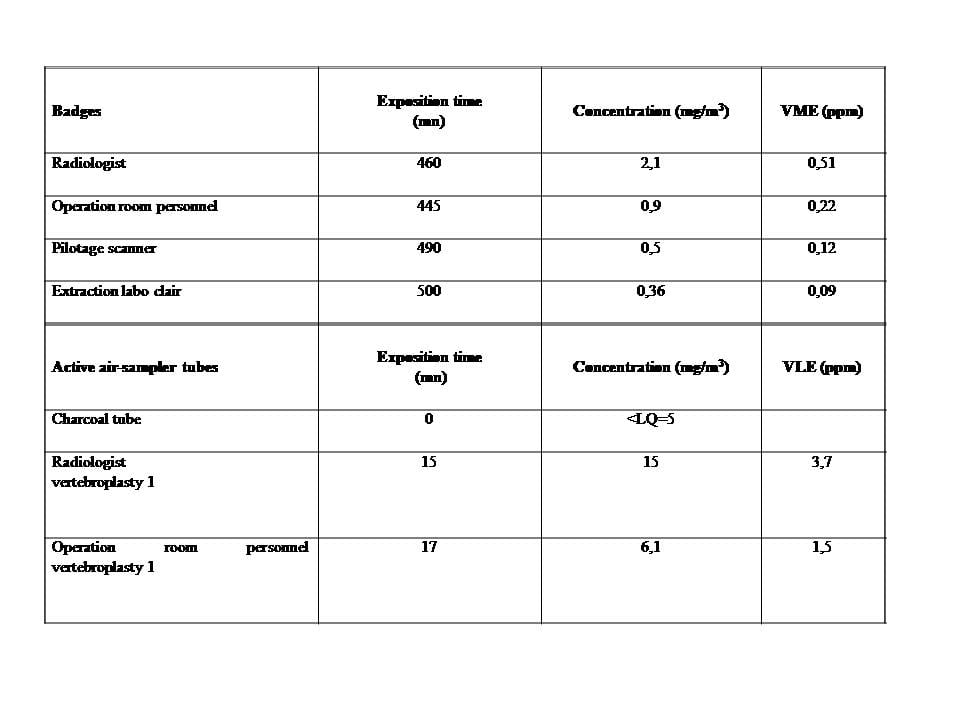
The mean MMA vapor concentrations obtained from the GABIE badge over the period of 460 min (Table 1) were 0.51 parts per million (ppm) for the radiologist and 0.22 ppm for the other operating room staff.
Mean MMA vapor concentrations were measured in the operating room near its filtering system at:
– 28 ppm following the mixing of PMMA
– 47 ppm following injection of PMMA.
It then dropped to concentrations inferior to 3 ppm.
The emission peaks measured by the activated charcoal tubes during the exposure time of 15 min were 3.7 ppm (Table 1 and Figure 1).
Discussion
The results of this study showed that MMA vapor concentrations during percutaneous vertebroplasty remained well below the published recommended standard of 100 ppm[4].This recommended exposure level is a time-weighted average for an 8-hour workday.
Because of its widespread use, there has been great interest in studying environmental safety and toxicity related to exposure to MMA vapors. Acute exposure to extremely high levels of MMA vapour can cause liver necrosis, pulmonary edema, and pulmonary emphysema[5]. Rats exposed to air containing 100 ppm of MMA for moret han 1 hour developed inter alveolar congestion and hemorrhage, as well as pulmonary vasodilatation and edema[6].
Very few studies have been published about exposure to MMA vapor concentrations during percutaneous vertebroplasty[21]. Cloft et al. [21]quantified MMA vapour levels in the samples by using gas chromatography during a period of approximately 1 hour corresponding to the duration of the vertebroplasty procedure: the ten samples obtained from the physician operators yielded MMA vaporl evels below the detection capability of the measuring technique, which was 4.88 ppm.
Our study confirmed that MMA vapour levels to which medical personnel
Were exposed during percutaneous vertebroplasty were well below the level typically considered hazardous. Moreover, our study attempted to further explore the exposure to MMA vapors on several points. First, the measurements were performed not only for the interventional radiologist but also for the other operating room staff. Moreover, they were performed in different locations: operating room personal, CT scan and near the filtration system of the operating room. Second, two techniques of sampling were used: a passive sampling method with GABIE badges to perform a long-term monitoring (i.e., over a period of 8 hours) and quantify the mean MMA vapor concentrations and an active sampling using gas chromatography and activated charcoal tubes to measure MMA vapour concentration peaks (i.e., during an exposure time of 15 min).It was shown that gas chromatography appeared to be more sensitive for detection of MMA fumes than photo-ionization detection[22].
The continuous measurement of MMA vapor concentrations during an 8-hour workday highlighted the fact that maximum vapor emission phase was between the time of mixing and the time of injection of PMMA. It dropped exponentially there after to negligible concentrations despite the persistent odor due to MMA fumes in the operating room. This decrease in MMA levels is probably due to airflow.
Moreover, a significant reduction in MMA vapor levels was also observed when the distance between the badge and the mixing bowl was greater. It was clearly shown by the difference of values between:
– first, the interventional radiologist preparing and injecting PMMA mixture and the radiologic technologists.
– secondly, the radiologist and the other locations(CT scan and near the filtering system of the operating room).
Even if the occupational exposure of medical personnel during percutaneous vertebroplasty remains clearly inferior to the levels necessary to elicit the main toxic effects, some side effects of inhaled MMA fumes might nonetheless occur. Dental students who were exposed to MMA vapour while preparing dentures complained of nausea and lack of appetite[23].MMA is known to be a potent allergenic sensitizer and can cause local reactions with dermal exposure[24]. It is also known to be a potential pulmonary toxin[6, 20]: a study of industrial workers with chronic exposure to MMA vapour levels of 9 to 38.5 ppm found that 20% of the workers had a chronic cough, versus only 1% of workers in a control group with no MMA exposure[20]. This study also found increased air way resistance as measured by spirometry in workers exposed toMMA vapor as compared with control subjects[20]. A case of asthma a induced by occupational exposure to MMA has also been reported[19].
Schlegel et al. [22]showed that the highest emission peaks were recorded during the mixing and preparation steps. Consequently, we used a mixing system instead of a traditional open container to reduce MMA exposure. The crucial role of mixing systems has already been shown in several studies[22, 25, 26]. In a first study, Schlegel et al. [22]found that vacuum mixing of bone cement with 7 commercially available mixing devices significantly reduced the emission of MMA vapors in the breathing zone when compared with classic hand mixing in an open bowl. In an effort to further reduce MMA exposure, the same authors[25]concluded that the latest generation of mixing devices which were pre-packed with the ingredients and thus allowed preparation in nearly closed circuits helped to reduce MMA exposure in the operating theatre. Moreover, Ungers et al. [26]compared the ability of two controlled preparation techniques with a traditional open container to reduce MMA emissions. One preparation technique affected a 73% reduction in measured MMA concentrations; the second one affected a 90% reduction. These authors[26]also indicated that these reductions were attributable to the preparation technique regardless of the type of cement being used.
Moreover,Wesley and Brinsko[11]strongly recommended that a filtering system should be used to lower the levels even further for both comfort and safety, which was the case in our study.
In conclusion, the operating room staff should be informed that despite the persistent odor due to MMA fumes, MMA vapour levels to which they are exposed during percutaneous vertebroplasty are wellbelow the level typically considered hazardous to the health.
2. Barragan-Campos HM, Vallee JN, et al. Percutaneous vertebroplasty for spinal metastases: complications. Radiology 2006;238:354-362.
3. Cotten A, Dewatre F, Cortet B, et al. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology 1996;200:525-530.
4. National Institute for Occupational Safety and Health. Methylmethacrylate: method 2537. In: NIOSH Manual of AnalyticalMethods. 4th ed. Cincinnati, OH: U.S. Department of Health and Human Services; 1995;1–4
5. Kessler MJ, Kupper JL, Brown RJ. Accidental methyl methacrylate inhalation toxicity in a rhesus monkey (Macaca mulatta). Lab Anim Sci 1977;27:388-390.
6. Raje RR, Ahmad S, Weisbroth SH. Methylmethacrylate: tissue distribution and pulmonary damage in rats following acute inhalation. Res Commun Chem Pathol Pharmacol 1985;50:151-154.
7. Lomax LG, Krivanek ND, Frame SR. Chronic inhalation toxicity and oncogenicity of methyl methacrylate in rats and hamsters. Food Chem Toxicol 1997;35:393-407.
8. Mizunuma K, Kawai T, Yasugi T, et al. Biological monitoring and possible health effects in workers occupationally exposed to methyl methacrylate. Int Arch Occup Environ Health 1993;65:227-232.
9. Bereznowski Z. Methacrylate uptake by isolated hepatocytes and perfused rat liver. Int J Biochem Cell Biol 1997;29:675-679.
10. Bereznowski Z. In vivo assessment of methyl methacrylate metabolism and toxicity. Int J Biochem Cell Biol 1995;27:1311-1316.
11. Wesley RE, Brinsko JD. Toxicity of methyl methacrylate monomer in orbital and cranial surgery. Ann Ophthalmol 1992;24:307-309.
12. Chan PC, Eustis SL, Huff JE, Haseman JK, Ragan H. Two-year inhalation carcinogenesis studies of methyl methacrylate in rats and mice: inflammation and degeneration of nasal epithelium. Toxicology 1988;52:237-252.
13. Donaghy M, Rushworth G, Jacobs JM. Generalized peripheral neuropathy in a dental technician exposed to methyl methacrylate monomer. Neurology 1991;41:1112-1116.
14. Hochman N, Zalkind M. Hypersensitivity to methyl methacrylate: mode of treatment. J Prosthet Dent 1997;77:93-96.
15. Lonnroth EC, Shahnavaz H. Use of polymer materials in dental clinics, case study. Swed Dent J 1997;21:149-159.
16. Blanchet LJ, Bowman DC, McReynolds HD. Effects of methyl methacrylate monomer vapors on respiration and circulation in unanesthetized rats. J Prosthet Dent 1982; 48:344-348.
17. McLaughlin RE, DiFazio CA, Hakala M, et al. Blood clearance and acute pulmonary toxicity of methylmethacrylate in dogs after simulated arthroplasty and intravenous injection. J Bone Joint Surg Am 1973;55:1621-1628.
18. Kirby BS, Doyle A, Gilula LA. Acute bronchospasm due to exposure to polymethylmethacrylate vapors during percutaneous vertebroplasty. AJR Am J Roentgenol 2003;180:543-544.
19. Lozewicz S, Davison AG, Hopkirk A, et al. Occupational asthma due to methyl methacrylate and cyanoacrylates. Thorax 1985;40:836-839.
20. Marez T, Edme JL, Boulenguez C, Shirali P, Haguenoer JM. Bronchial symptoms and respiratory function in workers exposed to methylmethacrylate. Br J Ind Med 1993; 50:894-897.
21. Cloft HJ, Easton DN, Jensen ME, Kallmes DF, Dion JE. Exposure of medical personnel to methylmethacrylate vapor during percutaneous vertebroplasty. AJNR Am J Neuroradiol 1999;20:352-353.
22. Schlegel UJ, Sturm M, Ewerbeck V, Breusch SJ. Efficacy of vacuum bone cement mixing systems in reducing methylmethacrylate fume exposure: comparison of 7 different mixing devices and handmixing. Acta Orthop Scand 2004;75:559-566.
23. Tansy MF, Benhayem S, Probst S, Jordan JS. The effects of methyl methacrylate vapor on gastric motor function. J Am Dent Assoc 1974;89:372-376.
24. Fries IB, Fisher AA, Salvati EA. Contact dermatitis in surgeons from methylmethacrylate bone cement. J Bone Joint Surg Am 1975;57:547-549.
25. Schlegel UJ, Sturm M, Eysel P, Breusch SJ. Pre-packed vacuum bone cement mixing systems. A further step in reducing methylmethacrylate exposure in surgery. Ann Occup Hyg 2010;54:955-961.
26. Ungers LJ, Vendrely TG, Barnes CL. Control of methyl methacrylate during the preparation of orthopedic bone cements. J Occup Environ Hyg 2007;4:272-280.
[/toggle]
GABIE Badges Exposure time
(min) Concentration (mg/m3) L-ELV (ppm)
Radiologist 460 2,1 0,51
Operating room staff 445 0,9 0,22
CT scan 490 0,5 0,12
Filtering system 500 0,36 0,09
Active air-sampling tubes Exposure time
(min) Concentration (mg/m3) S-ELV (ppm)
Radiologist 15 15 3,7
Operating room staff 17 6,1 1,5
Table. Passive sampling: atmospheric concentrations of MMAvapors were measured with GABIE badges attached to the surgical gowns of the interventional radiologist and the other operating room staff (two radiologic technologists) over a period of about 460 min. Two GABIE badges werealso positioned on the CT scan and near the filtering system of the operating room to measure MMA vapors concentrations in these locations. This passive sampling allowed to measure the long-term exposure limit values (L-ELV) corresponding to mean MMA vapors concentrations.
Active sampling was performed during 15 min using an individual pump placed near the breathing zone of the radiologist. MMA vapour levels in the samples were then quantified using ga schromatography and activated charcoal tubes(the detectioncapability of this measuring technique was 5 mg/m3). This active sampling allowed to measure the short-term exposure limit values (S-ELV)corresponding to emission peaks.
Table 1: Analysis of organic vapors and CO2 using an infrared spectroscopic detection before and after the use of MMA