Radiation dose to radiologists during dual guidance (CT and fluoroscopy) vertebroplasty and kyphoplasty: How much and where?
Abstract
Purpose
To quantify radiation exposure to operators performing vertebroplasty or kyphoplasty using combined fluoroscopy and CT guidance.
Material and Methods
The same operator performed 33 percutaneous vertebral reinforcements (24 vertebroplasties and 9 kyphoplasties) in 25 consecutive patients for the following indications: 16 osteopenic fractures, 7 malignant lesions, and 2 angiomas. Guidance was achieved using both fluoroscopy and repetitive acquisitions of 3 consecutive CT slices (so-called “smartstep mode”) on the target area. Radiation dose was measured on both orbits, both hands, both ankles, and under the lead-lined apron at the thorax level. CT irradiation was specifically quantified by a separate ionization chamber.
Results
Average radiation per procedure was: 0.45mSv for the right hand (0.67mSv for kyphoplasties, 0.37mSv for vertebroplasties; p<0.001), 0.2mSv for the left hand, 0.14mSv for the left orbit (0.23mSv for kyphoplasties, 0.11mSv for vertebroplasties; p<0.001), no measurable dose for the right orbit and both ankles (<0.1mSv, detector threshold). Fluoroscopy time positively correlated with dose to the left hand (p<0.01) and the left orbit (p<0.001). Effective CT dose was 0.0002mSv/procedure. CT acquisition time positively correlated with dose to the left orbit (p=0.01), and patient weight with dose to the left hand (p=0.03), left orbit (p=0.03), and thorax (p=0.02).Conclusion
Per-procedural CT guidance adjunction may be a valuable tool to reduce fluoroscopy use and in turn to reduce operator exposure, still achieving similar or superior treatment accuracy. Non-amenable factors, such as the procedure type and the patient’s weight, also influence the operator radiation.
Introduction
Each year in the United States of America over 700,000 vertebral fractures occur, among which 280,000 result in severe pain and 150,000 require hospitalization (1). Vertebroplasty and kyphoplasty are vertebral reinforcement procedures based on the injection of acrylic cement or bone substitute into the injured vertebral body. These therapeutic techniques can now be performed percutaneously with radiological guidance. Although these fluoroscopy-guided procedures improve therapeutic outcome (e.g. over 95% effectiveness for Garfin (2)), both the patient and medical team experience radiation exposure during the procedure. At our institution, we use a hybrid guidance technique combining fluoroscopy and a CT scanner to perform vertebropasty and kyphoplasty. These targeted CT acquisitions are intended to improve the accuracy of material positioning and safety of the procedure.
Several studies, which were not limited to spinal procedures, have evaluated the radiation exposure during fluoroscopic procedures with or without CT fluoroscopy (11, 14, 19). In the present study we evaluated the radiation dose received by the practitioner during a vertebroplasty and/or kyphoplasty performed in conjunction with the use of fluoroscopy and CT guidance tools. We evaluated radiation exposure at the following anatomical sites: orbits, hands, ankles, and thorax.Materials and methods
Design of the study
We conducted a prospective non-randomized study, including 25 consecutive patients over a 5-month period (August, 2006 through January, 2007) at our institution. This yielded a total of 33 percutaneous vertebral reinforcement procedures (24 vertebroplasties and 9 kyphoplasties). The study was approved by the local Institutional Review Board. Potential benefits of CT scanner guidance for reducing the radiation dose to the radiologist were evaluated.
The patients included 16 women and 9 men, aged 53-90 yrs (mean = 75 yrs) and weighing 40-100 kg (mean = 63.4 kg). The indications for treatment were osteopenic fracture in 16 cases, metastatic or myelomatous lesions in 7 cases, and aggressive angioma in 2 cases. 24 vertebroplasties were performed on 18 patients; two of them having vertebroplasties at 3 levels, and two other patients at two levels. Nine kyphoplasties were performed on 7 patients; two of them having kyphoplasties at two levels. The choice of the technique depended on the degree of vertebral compression, the delay of the lesion, and the etiology. Vertebropasties and kyphoplasties were equally distributed between thoracic and lumbar regions: 16 thoracic levels (12 vertebroplasties, 4 kyphoplasties) and 17 lumbar levels (12 vertebroplasties, 5 kyphoplasties).
We completed the procedure with an 8-row MSCT (Lightspeed 8, General Electric, Milwaukee, MI) and a fluoroscopy C-arm (Stenoscope C-arm, General Electric, Milwaukee, MI) positioned around the CT table, which were aligned with the target vertebra. The patient was lying in procubitus on the CT table, and the fluoroscopy detector was on the same side as the practitioner to the left of the patient. The same experienced and right-handed operator performed all interventions using the procedure described below.
The CT room is dedicated to intervention procedures and is also used as an operating room. The patients were prepped in a sterile fashion, and the region of intervention was covered with sterile fields. All equipment (CT, fluoroscopy, instrumentation table) within the intervention field was covered with sterile protection. Following regular scrubbing, the operator and assistants wore hair caps, breathing masks and surgical sterile gloves as well as gowns over their leaded pro-tection.
CT involved the acquisition of 3 consecutive slices on a selected region when judged necessary by the operator. The operator controlled the acquisition with a foot pedal after putting himself behind a leaded window. This mode of acquisition is labeled as “SmartStep mode” by the constructor of our CT scanner and is used for the guidance technique described below. CT images were displayed on a dedicated screen next to the fluoroscopy screen within the intervention room. CT acquisition parameters were the same for all procedures and were as follows: wide SFOV, voltage = 120 kV, charge = 60 mA, slice thickness = 7.5 mm, increment = 5 mm, three images/tube rotation (1 s).Radiation protection
Throughout the procedure, the operator wore a 5 mm thick lead-lined apron and a 5 mm thick lead-lined thyroid shield for fluoroscopy and CT radiation protection. Additionally, when performing per-procedural CT acquisitions (Smartstep mode) the operator stood behind a 2.5 mm lead equivalent thickness protective screen located 1 meter from the CT scanner table and close to the gantry, and at a height of 1.5 meters. This served to protect the operator’s upper body and face.Radiation Measurement
The measurement of irradiation (fluoroscopy and CT scanner) received by the operator was performed using six thermoluminescent dosimeters (TLDs) (+ 1 control) and one operational dosimeter. TLDs were carefully positioned on both orbits (at the level of the temples on the surgeon’s cap), both hands under the surgical gloves, and both ankles (on the trousers) of the operator. The control TLD was left in ambient light throughout the intervention in an adjacent room outside of the irradiation zone. The 7 TLDs were kept in darkness and away from any radiation before and after the intervention within the management office. The detection threshold for the TLDs was 0.1 mSv. This value was considered to be the minimum dose absorbed. An operational dosimeter (DMC 2000X, synOdys group, Lamanon, France) was placed under the lead-lined apron at the level of the thorax. To distinguish the irradiation due to the fluoroscopy from that originating from the CT scanner during guidance, the effective dose H*(10) linked to CT acquisition in SmartStep mode was evaluated using a high-volume ionization chamber (1,800 cm3) placed behind the lead-glass window at 1 meter from the exam table and at the right of the patient so as to simulate the operator’s position during the procedure.Procedure
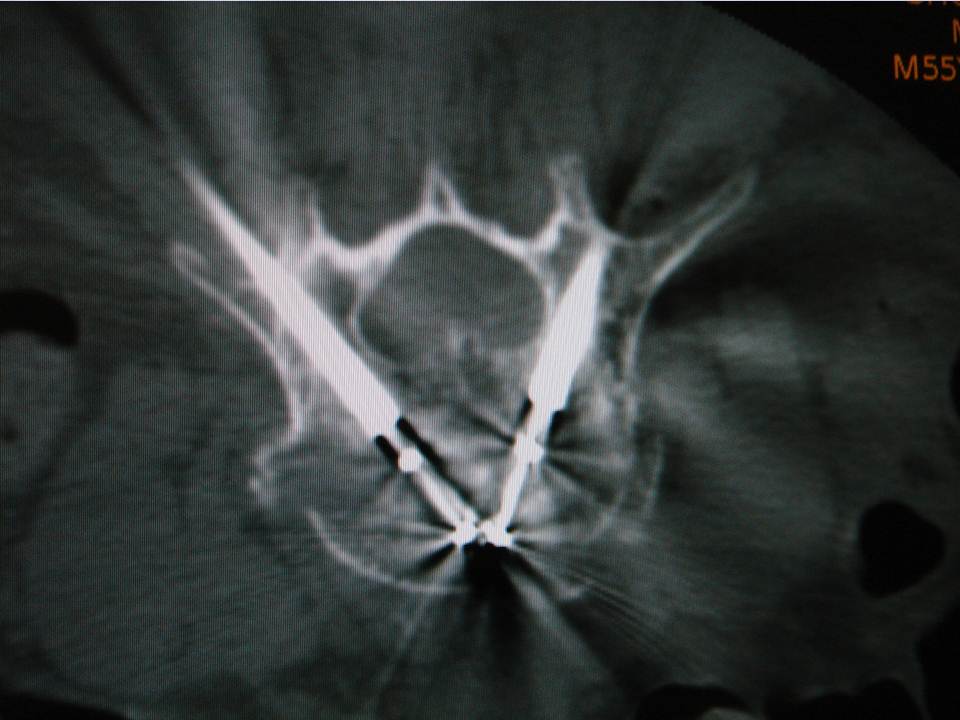
The guidance technique used was identical to that proposed by Gangi (4). It consists of pairing the use of targeted CT acquisitions during the procedure mode with fluoroscopy. CT acquisitions were not used for real-time guidance, but rather to check and improve needle/trocar trajectory planning, as well as cement delivery or balloon inflation. It essentially gives the same information as a regular helicoidal block acquisition, except there is much less coverage per acquisition (radioprotection). The command is also done by the operator himself directly from within the intervention room (intended to provide time efficiency). Fluoroscopy allows real-time verification during cement injection. When a kyphoplasty was needed, balloon inflation was performed under fluoroscopy with no additional use of the CT. The cement injection was performed under continuous fluoroscopy with additional SmartStep CT acquisitions in case there was any question of leakage. The procedure was immediately stopped in the event of epidural leaking. This dual monitoring combination was intended to improve the capability of detecting/avoiding the risk of cement leakage.Data gathering and statistical analyses
The patient data and acquisition parameters were recorded on the patient sheet and included: the CT and fluoroscopy acquisition parameters and their duration of use, the patient's weight, the target level, and the indication of the intervention, as well as the dose indicated at the end of the intervention on both the operator’s thoracic dosimeter and the ionization chamber. Statistical analyses were performed using SAS version 9.1.3 (Cary, NC, USA). We used a statistical significance threshold of p ≤ 0.05 as well as non-parametric statistical tests due to the small population and the nature of the variables: Fisher’s exact test for comparison of percentages, the Kruskall-Wallis test for the comparison of medians, Spearman’s rank correlation coefficient for the relationship between two quantitative variables. The hypothesis tests were two-tailed.Results
Irradiation exposure across anatomical sites
Table 1 shows the average dose values per anatomical site, as well as the duration of fluoroscopy and SmartStep procedures. For the patients with multi-level treatment, the irradiation values observed were divided by the number of levels. Results following vertebroplasties and kyphoplasties are first presented separately and then merged together. The TLDs had a detection threshold of 0.1 mSv. This value was therefore the minimum received dose. The dose recorded by the control sensor was < 0.1 mSv both for vertebroplasties and kyphoplasties. The operator’s right hand was the most exposed anatomical site with 0.45 mSv on average per procedure. This exposure significantly differed between kyphoplasty and vertebroplasty (0.67 mSv vs. 0.37 mSv; p<0.001). The main other sites subject to radiation were the left hand and the left orbit with 0.2mSv and 0.14 mSv on average, respectively. In the case of the left orbit, exposure was significantly higher for kyphoplasty vs. vertebroplasty (0.23 mSv vs. 0.11 mSv; p<0.001). The right orbit and the ankles did not receive any measurable radiation (< 0.1 mSv).Fluoroscopy and CT duration/irradiation
The vertebroplasty procedure required 1.93 min of fluoroscopy time on aver-age, and kyphoplasty significantly more fluoroscopy time with 3.75 min on average (p<0.001). The average fluoroscopy time for all procedures was 2.84 min. The effective dose due to CT Scanner in SmartStep mode has been measured at 15 nGy per series of 3 slices, or 15 nGy per second (3 slices/s). The average CT acquisition time in Smartstep mode was 11.4 s for the vertebroplasty procedures and 14.4 s for the kyphoplasty, yielding an average of 12.2 s (range: 6-36 s) per procedure. The effective dose per procedure was therefore 183 nGy (range: 90-540 nGy), which is equivalent to approximately 0.0002 mSv.
Table 2 demonstrates that the fluoroscopy time was significantly correlated with increased measured dose for the left hand (p=0.01) and for the left orbit (p<0.001). There was also a significant correlation between fluoroscopy C-arm milliamps and irradiation of the right hand (p=0.01) and the left orbit (p=0.007). Final-ly, the left orbit was the only anatomical site where increased CT acquisition time in SmartStep mode was positively and significantly correlated with increased radiation dose (p=0.01). Fluoroscopic time was similar for different levels, and no significant correlation was observed between the vertebral level treated and the radiation dose. Similarly, no correlation was found between the etiology for vertebroplasty or kyphoplasty treatment and the radiation dose. Patient weight was significantly correlated with increased radiation to the left hand (p=0.03), the left orbit (p=0.03), and the thorax (p=0.02)Discussion
Interventional radiology of the spine has grown significantly over the past several years. While improvements in terms of techniques and equipment have predominantly focused on reducing radiation exposure to patients (in part due to shortening the time of procedures), comparatively less attention has been given to the radiation dose received by the operator (2). This is particularly germane given 1) that shortened procedures per patient can result in increased numbers of procedures performed by a given operator and 2) that vertebroplasties and kyphoplasties are highly irradiating, compared with the irradiation exposure of coronary angioplasty (5). As another demonstration of this fact, a study of the activities of orthopedic surgeons showed that 90% of the effective dose received could be attributed to these procedures (6). In the present study, we addressed more precisely the dose to the operator when performing vertebroplasty or kyphoplasty, given the fact that we perform these procedures with a combined fluoroscopic and CT guidance. The results of our study confirm the relatively high overall level of irradiation received by the operator.
The irradiation sources can be divided into 2 main components: the primary beam, which may be crossed by the operator's hands during the intervention (7) and the scattered radiation from the patient, which constitutes 20-40% of the dose in the air of the incident radiation, to which it is added. In percutaneous vertebroplasty or kyphoplasty, irradiation doses to the operator are limited during the vertebral level selection and the positioning of the needle in contact to the periosteum, due to minimal fluoroscopic time. Irradiation is considered to be much higher during the insertion of the trocar into the vertebra, the positioning of the tamps, the vertebral expansion, and the injection of cement. During these steps, the operator is obliged to stay close to the patient and the irradiation source and must use fluoroscopy in continuous mode.
We showed that neither the targeted level nor the etiology of the vertebroplasty or kyphoplasty had a significant influence on radiation exposure. This reflects the fact that performing the procedure was unaffected by these two parameters. On the contrary, the irradiation dose depended on the initial parameters evaluated, in particular patient weight and fluoroscopy parameters. As expected, we found a significant correlation between the irradiation dose and fluoroscopy duration, reflecting both the quantity of primary-beam radiation and backscattered radiation.
The right hand, the left hand and the left orbit were the loci that were the most irradiated overall, with no discrimination between direct and scattered radiation, with cumulative irradiation doses of 0.45, 0.2, and 0.14mSv, respectively. The left hand, the left orbit and the thorax radiation doses were significantly correlated with the patient’s weight, showing the influence of scattered radiation for these loci, which were also the ones closest to the patient. It should be noted that the right hand was the most irradiated site. One explanation might be that it was the closest to the fluoroscopy x-ray tube during the irradiating steps, in particular that of cement injection (particularly given that the operator in this study was right-handed). Supporting this explanation, the right hand irradiation (but not the left hand) was also significantly correlated with the C-arm milliamps.
The dose delivered to the operator under the SmartStep CT mode was ex-tremely low (0.0002 mSv). One SmartStep acquisition was only three slices, and the equivalent of about 40 slices were on average acquired per procedure. In addition, the operator was behind a leaded window and over 1.5 m from the gantry during these acquisitions. These factors can explain the nearly negligible dose. Nevertheless, we surprisingly observed a correlation with the left orbit radiation dose and the Smartstep duration acquisition. At present, we have no straightforward explanation for such. There were two goals in using the CT guidance in Smartstep mode (i.e. as opposed to fluoro-CT in real time): 1) to benefit from the volumetric data given by a CT acquisition for positioning material accurately and for maintaining safety during the various steps of the procedure, and 2) to reduce overall irradiation dose. We think that the gain in accuracy allowed by CT slice acquisitions resulted in faster positioning of the needles, trocar, balloons and cement. Consequently, this reduced the need for fluoroscopy. The possibility to check the exact filling of cement or balloon inflation likely also led to a savings in terms of some fluoroscopic time by reducing the uncertainty during these steps. With 1.93 min and 3.75 min of fluoroscopy time for vertebroplasty and kyphoplasty, respectively, the addition of CT guidance seems actually to have reduced the fluoroscopy time needed when compared to data of vertebroplasty/kyphoplasty conducted with fluoroscopy alone. Except for the study of Harstall et al. (8) where mean vertebroplasty fluoroscopy time was 1.34min, other teams using fluoroscopy alone have a fluoroscopy time ranging from 5.4 to 60 minutes.
Komemushi’s study evaluated fluoroscopy time using fluoroscopy and CT guidance (16). CT guidance was, however, different than the SmartStep technique we used here, although not used as a fluoroscopic CT. They obtained a fluoroscopy time ranging between 6.66 min and 7.54min (8), which is also in the lower range of values when compared to using fluoroscopy alone. Our data suggest that the radiation dose to the operator related to CT was nearly negligible. Therefore, if the use of CT can achieve significant decreases in fluoroscopy time, it would appear to directly result in significant decreases in overall radiation dose to the operator. This does not mean than the overall dose to the patient decreases in the same proportion, because the radiation of CT acquisition would have to be taken into consideration, and this was not the scope of the present study.
In our experience, SmartStep mode guidance is more accurate than fluoroscopy alone. This is because the radiologist can both adjust the needle’s trajectory more precisely and easily than with fluoroscopy alone and also check quickly and easily each step of the procedure as needed, rather than being restricted to one single CT acquisition at the start of the procedure. It is true that this technique does not allow real-time guidance of the needle (13) as is the case when using the fluoroscanner in continuous mode. However, it is reasonable to say that it constitutes a reasonable compromise between guidance accuracy and radiation exposure in view of the major difference in irradiation with the fluoroscanner in continuous mode. Effectively, using the CT scanner in continuous fluoroscanner mode can achieve irradiation doses as high as 1 mGy/sec (10-12). Paulson also showed the advantage of multiple targeted CT acquisitions as we did here, when compared to fluoroscopy alone or CT fluoroscopy for radiation purposes (14)
Finally, another parameter potentially affecting fluoroscopy duration (and therefore operator irradiation) was the operator’s experience. All the procedures were performed by a single operator with extensive experience in this type of intervention, which we believe also resulted in more homogeneous data. Gianfelice (15) showed that the fluoroscopy duration is reduced by 50% after 200 procedures under fluoroscopy and CT guided interventions.Impact of Irradiation on Activities
This study allowed us to extrapolate the maximum number of procedures that can be performed per year by an individual operator. According to French Decree No. 2003-296 of March 31, 2003 concerning the protection of workers against the dangers of ionizing radiation, the maximum authorized total equivalent dose limits per year are 500 mSv for hands and 150 mSv for the lens. Consequently, the average irradiation values for the right hand limits the number of procedures to 746 Kyphoplasties or 1351 Vertebroplasties per year, respectively, or 1111 per year for a mixture of the two procedures. The average irradiation values for the left orbit limits the number of procedures to 652 Kyphoplasties or 1363 Vertebroplasties per year, respectively, or 1071 per year for a mixture of the two procedures.
The irradiation to the right hand and the left orbit would also limit the number of procedures per year to almost the same extent, despite the fact that the irradiation of the left orbit was lower. This is because of differences in the maximum authorized doses at each locus. In comparison with other procedures, Ramsdale (19) showed that the radiation doses received by the radiologist undertaking biliary and renal interventional techniques ranged from 0.27 to 1.29 mSv per examination.Limitations
All the vertebroplasties and kyphoplasties were performed with fluoroscopy and CT guidance. We therefore cannot differentiate the relative proportion of radiation dose to the operator due to fluoroscopy versus CT, although our data would suggest that CT’s component is minimal. It would likewise have been interesting to determine the radiation dose had the procedures been performed with fluoroscopy alone, without the help of CT guidance and with all other technical aspects being identical. This would have lead to quantification of the added value - if any - of SmartStep mode CT guidance regarding radiation dose. Because actual techniques in vertebroplasties and kyphoplasties can be very different across centers or practitioners, a true comparison is nearly impossible, even when the treatment paradigm is the same. This makes it challenging to isolate the effect of one step/tool of a procedure on the radiation dose. As an example, some techniques use veinography with continuous fluoroscopy, which lengthens fluoroscopy time and increases irradiation (8, 17). The use of a cement injector is another example where modifying injection time paradoxically results in a longer injection duration and thus associated fluoroscopy time (18, 19).
Conclusions
Radiology-guided vertebroplasty and kyphoplasty reinforcement procedures are becoming increasingly widespread and are now performed in most university hos-pital centers, using radiological guidance that differs across teams. Combined real-time fluoroscopy and CT scanner guidance through repeated targeted acquisitions (e.g. SmartStep mode) is used in our institution and resulted in reduced irradiation for the practitioner due to reduced fluoroscopy time and negligible dose from CT acquisitions. This offers a reasonable alternative to fluoro-CT with the advantages of procedure accuracy and effectiveness as well as limited operator’s radiation. The latter is the highest at the operator’s right hand, followed by the left hand and left orbit and is essentially correlated with the fluoroscopy time. This demonstrates the need for highly efficient procedures limiting fluoroscopy. However, factors such as patient weight and fluoroscopy parameters influence the operator’s irradiation as well, but not the level treated or the etiology for treatment. Also, a full understanding of irradiation topography is required if we want to develop efficient strategies to decrease effective operator irradiation to relevant anatomical areas, as it directly impacts the number of procedures per year that can be performed and by extension health authority policies.
[toggle title="REFERENCES"]1 Garfin SR, Reilley MA. Minimally invasive treatment of osteoporotic vertebral body compression fractures. Spine J 2002; 2:76–80.
2 Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine 2001; 26: 1511–1515.
3 Vertebroplasty and kyphoplasty: do fluoroscopy operators know about radiation dose, and should they want to know? Radiology. 2004 Sep;232(3):633-4.
4 Gangi A, Kastler BA, Dietemann JL. Percutaneous vertebroplasty guided by a combination of CT and fluoroscopy. AJNR Am J Neuroradiol 1994;15(1):83-6.
5 Karppinen J, Parvianinen T, Servomaa A, et al. Radiation risk and exposure of radiologists and patients during coronary angiography and percutaneous transluminal coronary angioplasty (PTCA). Radiat Prot Dosimetry 1995;57:481–485.
6 Theocharopoulos N, Perisinakis K, Damilakis J, et al. Occupational exposure from common fluoroscopic projections used in orthopaedic surgery. J Bone Joint Surg Am 2003;85:1698–703.
7 Goldstone KE, Wright IH, Cohen B. Radiation exposure to the hands of orthopaedic surgeons during procedures under fluoroscopic X-ray control. Br J Radiol. 1993 Oct;66(790):899-901.
8 Harstall R, Heini PF, Mini RL, Orler R. Radiation exposure to the surgeon during fluoroscopically assisted percutaneous vertebroplasty: a prospective study.Spine. 2005 Aug 15;30(16):1893-8.
9 Komemushi A, Tanigawa N, Kariya S, Kojima H, Shomura Y, Sawada S. Radiation exposure to operators during vertebroplasty. J Vasc Interv Radiol. 2005 Oct;16(10):1327-32.
10 Katada K, Kato R, Anno H, et al. Guidance with real-time CT fluoroscopy: early clinical experience. Radiology 1996;200:851-856.
11 Neeman Z, Dromi SA, Sarin S, Wood BJ. CT Fluoroscopy shielding: decreases in scattered radiation for the patient and operator. J Vasc Interv Radiol 2006;17:1999-2004.
12 Silverman SG, Tuncali K, Adams DF, Nawfel RD, Zou KH, Judy PF. CT fluoroscopy-guided abdominal interventions: techniques, results, and radiation exposure. Radiology. 1999 Sep;212(3):673-81.
13 Chiras J, Depriester C, Weill A, Deramond H. Vertebroplasties percutannées : Technique et indications. J. Neuroradiol 1997;24:45-59.
14 Paulson EK, Sheafor DH, Enterline DS, McAdams HP, Yoshizumi TT. CT fluoroscopy-guided interventional procedures: techniques and radiation dose to radiologists. Radiology. 2001 Jul;220(1):161-7.
15 Gianfelice D, Lepanto L, Perreault P, Chartrand-Lefebvre C, Milette PC. Effect of the learning process on procedure times and radiation exposure for CT fluoroscopy-guided percutaneous biopsy procedures. J Vasc Interv Radiol. 2000 Oct;11(9):1217-21.
16 Ramsdale ML, Walker WJ, Horton PW. Extremity doses during interventional radiology.Clin Radiol. 1990 Jan;41(1):34-6.
17 Mehdizade A, Lovblad KO, Wilhelm KE, Somon T, Wetzel SG, Kelekis AD, Yilmaz H, Abdo G, Martin JB, Viera JM, Rüfenacht DA. Radiation dose in vertebroplasty. Neuroradiology. 2004 Mar;46(3):243-5.
18 Kallmes DF, O E, Roy SS, Piccolo RG, Marx WF, Lee JK, Jensen ME. Radiation dose to the operator during vertebroplasty: prospective comparison of the use of 1-cc syringes versus an injection device. AJNR Am J Neuroradiol. 2003 Jun-Jul;24(6):1257-60.
19 Ortiz AO, Natarajan V, Gregorius DR, Pollack S. Significantly reduced radiation exposure to operators during kyphoplasty and vertebroplasty procedures: methods and techniques. AJNR Am J Neuroradiol. 2006 May;27(5):989-94.[/toggle]